Abbott Instinct CGM to Work with Medtronic 780G Under FDA Nod
Once considered competitors in diabetes technology, Medtronic and Abbott are now working together to expand access and choice for patients.
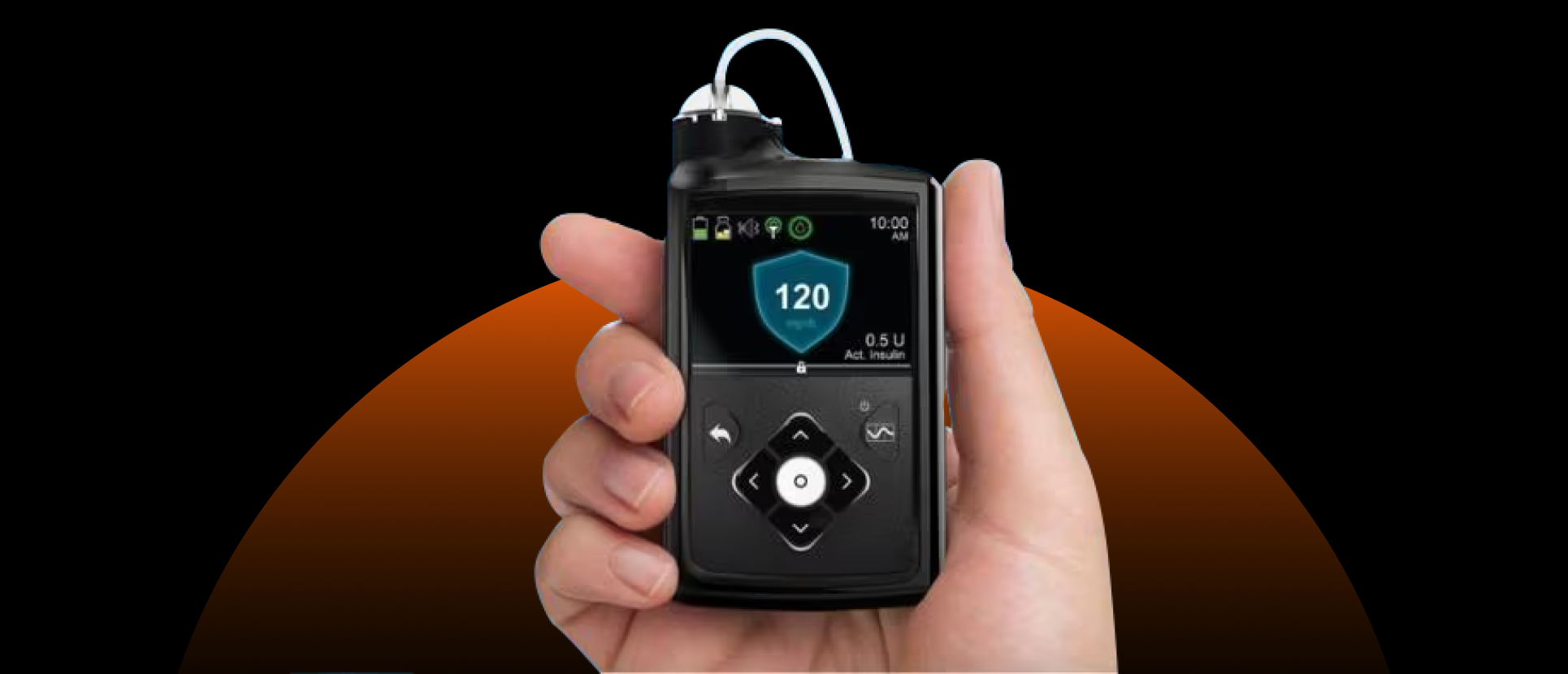
The US Food and Drug Administration (FDA) has approved the integration of Medtronic’s MiniMed 780G system with Abbott’s Instinct continuous glucose monitor (CGM), marking a significant step in diabetes management technology.
The approval allows interoperability between the MiniMed 780G automated insulin delivery system and Abbott’s Instinct CGM sensor, designed to provide seamless glucose monitoring and insulin control. It also expands the use of the MiniMed 780G to adults with type 2 diabetes.
The collaboration gives patients multiple CGM options within a smarter dosing ecosystem, offering greater flexibility in managing diabetes.
Abbott’s Instinct is described as the world’s smallest and thinnest integrated CGM, designed for 15-day wear. Medtronic’s MiniMed 780G is the first automated insulin delivery system with Meal Detection technology available for type 2 diabetes.
Industry analysts note that the combined ecosystem is expected to reduce the daily management burden for patients while reshaping the competitive landscape of diabetes care.
Once considered competitors in diabetes technology, Medtronic and Abbott are now working together to expand access and choice for patients.










